Pre-Feasibility study of Production Disposable Medical Devices
In Pre-Feasibility study of Production Disposable Medical Devices Technical, Financial, and Economic aspects of the project are explained .
As healthcare standards and practices improve, the shift from reusable to disposable medical devices has been unstoppable. Devices that were washed, disinfected, and sterilized in hospitals just a decade ago are now available in more affordable single-use forms. These devices cover the full range of care provided to the patient. From simple examinations to highly specialized procedures, doctors use them for each patient.
The importance of the project
Considering the current market climate in the country , population growth, a changing epidemiology, a growing medical tourism industry, healthcare infrastructure developments, expanding health insurance throughout the region, digital transformation, and new technologies, the need for medical devices is increasing exponentially. Added to the opportunities available in the region for manufacturing, including the access to affordable raw materials and energy and the young educated workforce, this growing demand makes production of these medical devices a profitable necessity.
Technical Specifications| Pre-Feasibility study of Production Disposable Medical Devices
The main equipment and devices for the production of all kinds of disposable medical products include extruders, injection machines, along with the required molds for the production of product parts. According to the various processes of production of parts, automatic assembly lines are used to assemble the parts needed to produce the final product, and finally, the sterilization of the packaged products is done using PLC and balanced monitoring of production lines. Parts of the syringe parts, such as coated needles, are purchased ready-made from suppliers. The packaging and sterilization department and the quality control tools of the products produced using modern production technologies are the main components of the production line that follow GMP standards.
Product Classification | Pre-Feasibility study of Production Disposable Medical Devices
Medical devices have many classifications based on different criteria. But from a production point of view there are three classifications that are helpful in decision making for starting a new factory:
- Classification Based on kind of Raw Materials such as: Petroleum Based Polymers, Metals, etc.
- Classification Based on Speciality and use such as: General, Surgical, etc.
- Classification Based on Production Technology level: medium, Advanced, etc.
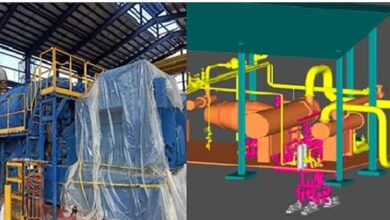
Automatic Manufacturing and Production Process
Manufacturing Facilities are Equipped with the Best Technologies. The production process is designed to avoid any possible mistake. From needle manufacturing to final stage of packaging we manufacture our products with fully automatic robotic machineries. Through several different logs and batch information tracing finished products has become possible. Unique in-house & customized machineries have enabled us to manufacture the best medical devices has been ever made.
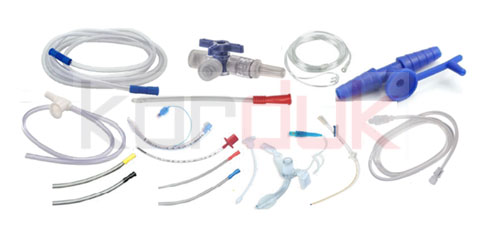
Products| Pre-Feasibility study of Production Disposable Medical Devices
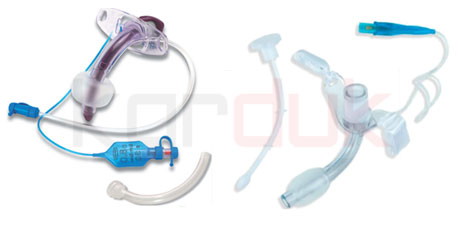
Heparin Cap
Sterile Heparin Cap for Single Use Easy and convenient in handling, close needle-free injection sites and offer a needle access via latex-free membrane
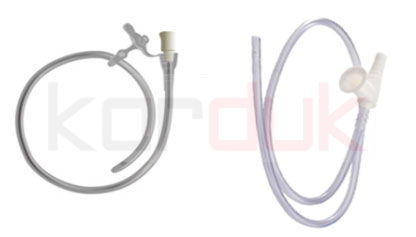
Feeding Tubes
A feeding tube is a plastic tube that is used to bypass chewing and swallowing in a patient who is not able to eat or drink safely. These tubes can be used to deliver both food and fluids, and can also be used for providing medications when needed. A feeding tube can also be used to remove fluids from the stomach if the body isn’t processing stomach contents well.
Nasogastric Tubes | Pre-Feasibility study of Production Disposable Medical Devices
A nasogastric tube is a narrow bore tube passed into the stomach via the nose. It is used for short- or medium-term nutritional support, and also for aspiration of stomach contents, for decompression of intestinal obstruction. A wide-bore tube is used if drainage is needed; otherwise, a finer bore tube is used. Fine-bore feeding tubes (gauge less than 9) cause less discomfort and less risk of rhinitis, pharyngitis or esophageal erosion. The use of a nasogastric tube is suitable for enteral feeding for up to six weeks. Polyurethane or silicone feeding tubes are unaffected by gastric acid and can therefore remain in the stomach for a longer period than PVC tubes, which can only be used for up to two weeks. For long-term enteral feeding, the use of percutaneous endoscopic gastrostomy (PEG) is associated with improved survival, better tolerance by the patient and lower incidence of aspiration.
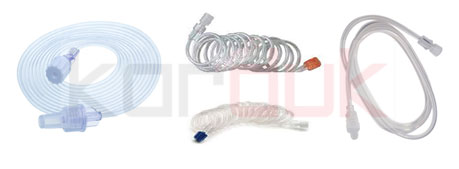
Suction Tubes
Amongst the instruments and machines which surgeons make use of while performing a surgery, suction tubes are one of them. These suction tubes are attached to a machine which provides suction to the surgeon when it is necessary.
The process of suction is used to get rid of any liquids which may come in the surgical area where the operation is being performed. Suction tubes are used in all areas of surgery and come under surgical instruments, dentistry instruments as well as gynecological instruments.
These suction tubes are not only found in the operation room but they are also found in an ambulance in case any liquids need to be cleared away from a patient in a state of emergency. These tubes attached to suction machines are also found in clinics and hospital rooms.
The suction tubes each perform a particular application which is why the suction machine has a number of suction tubes attached to it. similar to all other kinds of surgical and gynecological instruments, the suction machine is made in such a way that the tubes and their tips can be replaced with ease so that the tubes can be used interchangeably between patients.
3 Way Stopcock (with Protectors)
stopcock is used for Infusion Therapy and Pressure Monitoring Line. Rotating male luer lock and fully threaded female ports ensure a safe and secure connection with other components. Straight internal flow channels facilitate a non-obstructive fluid flow with an optimized internal diameter for accurate pressure monitoring. Rigid construction, no internal fluid meniscus and no air trap within the housing.
Fingertip for Suction Tubes | Pre-Feasibility study of Production Disposable Medical Devices
Fingertip for Suction Tubes are made of durable and shatter-resistant plastic and are available in four different types. They are lightweight and transparent, allowing for easy observation of flow. The connectors are supplied in convenient dispenser cartons that are clearly labelled by type.
Male Nelaton Catheters
A range of single use, disposable intermittent catheters for male use. They are made of transparent high quality medical grade PVC and come in a sterile peel pack. Available in various lengths and degrees of firmness.
The nasal cannula | Pre-Feasibility study of Production Disposable Medical Devices
(NC) is a device used to deliver supplemental oxygen or increased airflow to a patient or person in need of respiratory help. This device consists of a lightweight tube which on one end splits into two prongs which are placed in the nostrils and from which a mixture of air and oxygen flows.
Suction Catheters
A suction catheter is a medical device used to extract bodily secretions, such as mucus or saliva from the upper airway. A suction catheter connects to a suction machine or collection canister
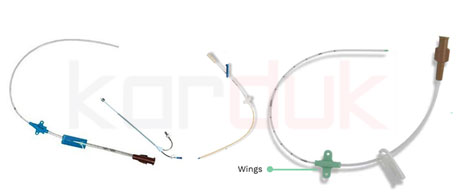
Cutdown Catheter | Pre-Feasibility study of Production Disposable Medical Devices
Venous cutdown is an emergency procedure in which the vein is exposed surgically and then a cannula is inserted into the vein under direct vision. It is used to get vascular access in trauma and hypovolemic shock patients when peripheral cannulation is difficult or impossible. The saphenous vein is most commonly used.
Rectal Tubes
Rectal tubes may be safely and effectively used to prevent soiling in critically ill patients with diarrhea. The use of rectal tubes as adjuncts to healing and preventing pressure sores in criti cally ill patients merits further study.
Extension Tubes | Pre-Feasibility study of Production Disposable Medical Devices
The PVC extension tubes are used as connection tubes for linear dosers for intravenous or intra-arterial infusion therapy. Termination of the tube on both ends with Lure Lock connectors allows safe connection to standard syringe for linear doser and to intravenous or intraarterial catheter.
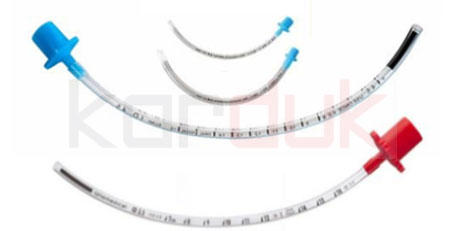
Endotracheal Tubes with Cuff
The Endotracheal Tube (ETT) is inserted through the mouth or nose into the trachea and connected to a ventilation device to
Endotracheal Tubes without Cuff
Tracheostomy Tubes with Cuff
A tracheostomy (trach) tube is a curved tube that is inserted into a tracheostomy stoma (the hole made in the neck and windpipe . There are different types of tracheostomy tubes that vary in certain features for different purposes
The ISIC code of product | Pre-Feasibility Studies of Disposable Medical Devices
The International Standard Industrial Classification of All Economic Activities (ISIC) is the international reference classification of productive activities. Its main purpose is to provide a set of activity categories that can be utilized for the collection and reporting of statistics according to such activities.
According to the ISIC classification system, the ISIC code (3311 ) is related to the production of various Disposable Medical Devices. And the ISIC code of the products of this plan is as follows
- Packaging of disposable medical equipment 7495412402
- Disposable medical devices 33111490
- Hospital disposables 33111643
- Medical instruments and devices 33111410
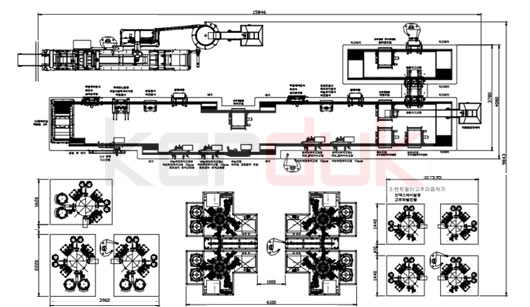
Buildings required for the project implementation
The building space required for the production, administrative and headquarters departments, according to the site plan and layout of production Disposable Medical Devices , is as follows:
- Industrial production area include cleanroom 1200 Mr2
- GMP Storage Rooms for Raw Materials and Finished Products 1000 Mr2
- Mechanical and Electrical facilities 500 Mr2
- laboratory: 150 Mr2
- Office Building and Conference Rooms 250 Mr2
- Staff Welfare Areas 200 Mr2
- Mechanical and Electrical facilities and boilers : 150 Mr2
- Security Building : 50 Mr2
- GMP Entrance Areas : 200 Mr2
Disposable Medical Devices production process
Disposable medical equipment production lines include three sections: a section for producing molded parts from medical plastic and PVC pipes, an assembly section according to the platform of each product, and a final packaging and sterilization section.
In the product production process, first the main granules, along with the masterbatch if needed, are fed to the injection machine after degassing, and the polymerization process and product shaping are carried out under pressure and heat in the screw cylinder and mold of the machine
The production environment, process, and products follow GMP standards, and the injection molding machines and extruders for pipe production and its molds are completely made according to the requirements for the production of medical equipment. In the production process, the production parts are placed on the assembly lines of each product.
The product assembly production lines in the feeding section have circular trays that allow the parts to be transferred to the assembly line by suction and vibration. Based on the production planning and the deployment plan of machines with practical capacity, the parts and tubes are cut, connected, and fixed, and the product is prepared for packaging. And with the monitoring system and quality control equipment before and during production, optimal production is possible until the end of the production line.
The packaging machine is specifically designed for packaging using high-strength polyolefin films only and is manufactured in accordance with the requirements and standards of the GMP medical and pharmaceutical industries.
Raw materials, auxiliary materials and packaging
The main raw materials of the design are heavy polyethylene, polypropylene and PVC. Due to their chemical structure, these materials do not tend to react with drugs and other liquids. Therefore, they can be used in the field of manufacturing products.
Medical grade polypropylene GA730 is used to produce the body and medical grade polyethylene is used to manufacture moving parts. The raw material for producing the sheets is medical grade PVC. The raw materials used in the manufacture must be free of any additives and impurities, known as medical grade, which can be supplied from domestic petrochemicals. Ethylene oxide is also required to sterilize the products.
Introduce Target Market | Pre-Feasibility study of Production Disposable Medical Devices
Currently, there is an effective demand in hospital sectors for appropriate value-added products. The main target market of this project, technically and economically, at the national level, using modern marketing methods and networks, in the first stage, is to meet the country’s needs and export. The ultimate goal of the project is to gain a one percent share of the domestic market with the possibility of exporting from the second year of production.
Financial Projection| Pre-Feasibility Studies of Disposable Medical Devices
The financial statements and operating costs of Production Disposable Medical Devices show a suitable situation for the project, considering the cost price of the products and the annual profit. The results of the analysis performed by the Comfar software, with a discount rate of 25%, also show an acceptable situation in terms of financial and economic terms for the base Disposable Medical Devices project with a capacity of 90000 tons per year.
Summary of study Results
Production calculations show that the proposed production capacity can be increased according to the needs of the domestic market and the country, considering the competitive price of the products compared to foreign products.
More than a decade of executive experience of the shareholders’ team in the industries and applications of medical equipment and effective communication with the sales market network can guarantee the success of this activity.
For more information about the Feasibility Studies of Disposable Medical Devices. you can contact Karduk.INC via WhatsApp, Telegram or E-mail.
Phone: +982166418908
Email: karduuk@gmail.com